Background
Double-hit (DH) lymphoma or triple-hit (TH) lymphoma, according to 2016 WHO Classification Criteria, is recognized as a distinct subset of non-Hodgkin lymphoma. The optimal treatment for DH/TH lymphoma remains unclear, although outcomes are inadequate with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) alone. Selinexor is a first-in-class, oral therapeutic that forms a slowly reversible covalent bond with cytoplasm by exportin 1 (XPO1/CRM1), leading to its inactivation. The U.S. Food and Drug Administration has approved selinexor monotherapy for relapsed/refractory DLBCL. Previous studies also suggested that selinexor was used to treat 5 patients with relapsed/refractory DH lymphoma, and 1 patient achieved CR and 2 achieved PR. Here, we report on the efficacy and safety of selinexor in combination with RCHOP as first-line treatment in patients with DH or TH lymphoma.
Methods
This prospective, phase 2, exploratory study was conducted in Zhejiang Cancer Hospital. The primary endpoint of the study was to evaluate the objective response rate (ORR), including complete remission (CR) rate and partial remission (PR) rate. In addition, diagnostic tumor tissue was prepared to conduct NGS analysis, and dynamic peripheral blood samples were collected before treatment, after 4 cycles when achieving CR from first-line chemotherapy, and progression of disease, to conduct ctDNA analysis. The initial dose of each drug in this regimen is as follows (repeat every 21 days, 6 cycles in total): rituximab: 375mg/m 2, d0; vindesine 4mg, d1; epirubicin 75mg/m 2, d1 (or liposomal doxubicin 35mg/m 2, d1); cyclophosphamide: 750mg/m 2, d1; prednisone: 100mg, d1-5; selinexor 60mg orally once a week. AEs were assessed according to the National Cancer Institute Common Terminology Criteria (CTCAE, version 4.0).
Results
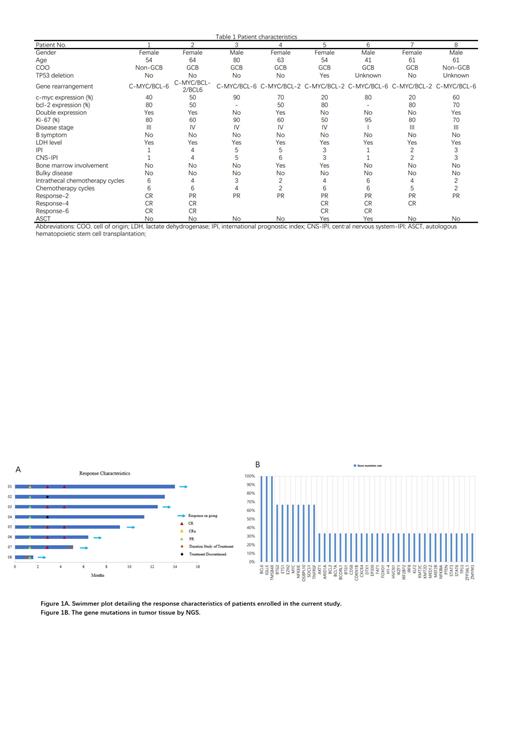
From May, 2022, 8 patients were enrolled in the study, including 5 females and 3 males. All patients had received at least 2 cycles of selinexor with RCHOP. A full summary of patient baseline characteristics and response to chemotherapy was shown in Table 1. Four patients have completed full cycles of chemotherapy, while 2 patients are still undergoing treatment, and 2 patients suspended subsequent chemotherapy due to rejection of antitumor treatment. A total of 34 cycles of chemotherapy have been completed. Best responses after induction as assessed by positron emission tomography/CT were 5 CRs and 3 PRs. Two patients received ASCT as consolidation therapy after achieving CR. CNS prophylaxis with intrathecal methotrexate was administered to all patients for 2-6 cycles. No patient experienced disease progression, and no patient had CNS involvement during follow-up period (Figure 1A). All patients were assessable for toxicity. All patients in the study received prophylactic granulocyte colony-stimulating factor after each cycle of chemotherapy. The most common hematologic toxicities included anemia (15/34, 44.1%), neutropenia (20/34, 58.8%), thrombocytopenia (13/34, 38.2%), leukopenia (10/34, 29.4%). Other nonhematologic toxicities included fever (6/34, 17.6%), nausea (16/34, 47.1%), vomiting (13/34, 38.2%), fatigue (20/34, 58.9%), and infection (5/34, 14.7%). NGS was conducted successfully in 3 patients, while another 5 patients could not provide NGS data due to insufficient tumor tissue. 139 somatic alterations were detected in 3 patients, with a median of 30 mutations per sample. BCL6/IGLL5/TMSB4X were the most frequently mutated gene in 100% of patients followed by 66.7% who had BTG2/ETS1/EZH2/MYC/NFKBIE/OSBPL10/SOCS1/TNFRSF14 mutations. TP53 mutations were detected in 33.3% of the patient (Figure 1B). In 71.4% (5/7) of the cases, we were able to detect a total of 143 variants in the ctDNA from pretreatment serum samples. 80 variants were detected in both tumor tissue and peripheral blood samples. 4 variants were detected only in the ctDNA, and 59 were detected only in the tissue biopsy. Serial ctDNA monitoring results showed that 100% (8/8) reduction to zero in ctDNA after 4 cycles of chemotherapy.
Conclusions
Selinexor combined with RCHOP may be an effective first-line regimen for patients with DH/TH lymphoma. Dynamic testing of ctDNA demonstrated deep remission to chemotherapy. Based on the encouraging results of the current study, a large scale, multicenter trial is needed to further investigate this promising regimen.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal